learn more
Dr. Thomas Blevins of Texas Diabetes and Endocrinology discusses this
new medication and everything we know about it so far.
November 8, 2023, the FDA approved the injectable GIP/GLP-1 dual incretin-based agonist tirzepatide for chronic weight management among adults with a BMI of 30 kg/m2 or higher or those with a BMI of 27 kg/m2 or higher plus one weight-related condition, such as high blood pressure, type 2 diabetes, or higher cholesterol. Tirzepatide helped people who were overweight or obese and did not have diabetes to lose about 18% of their body weight compared to people receiving a placebo. Tirzepatide is already approved for type 2 diabetes under the name Mounjaro, and its effectiveness in those patients is due in part to its weight loss effects.

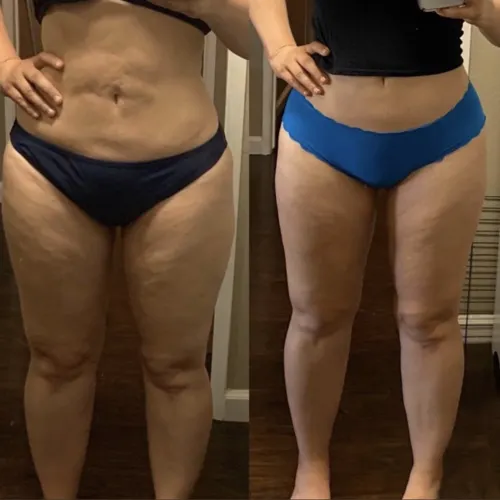
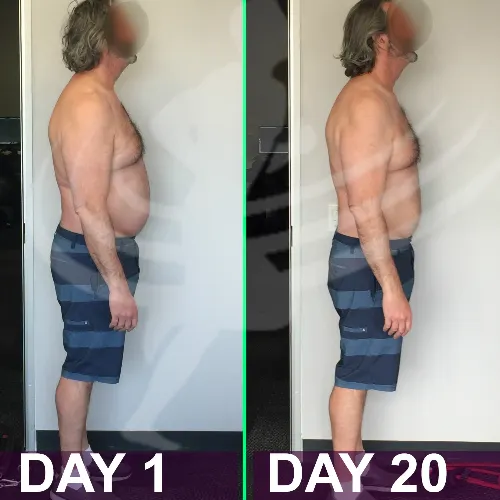
See the Difference
Don't wait any longer, this is your opportunity to change your life today. Never look back and know Empower Health Peptide Wellness has you covered.

This web site is provided for educational and informational purposes only and does not constitute providing medical advice or professional services. The information provided should not be used for diagnosing or treating a health problem or disease, and those seeking personal medical advice should consult with a licensed physician. Always seek the advice of your doctor or other qualified health provider regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on UW Medicine’s website. If you think you may have a medical emergency, call 911 or go to the nearest emergency room immediately. No physician-patient relationship is created by this web site or its use. Neither the University nor its employees, nor any contributor to this web site, makes any representations, express or implied, with respect to the information provided herein or to its use.